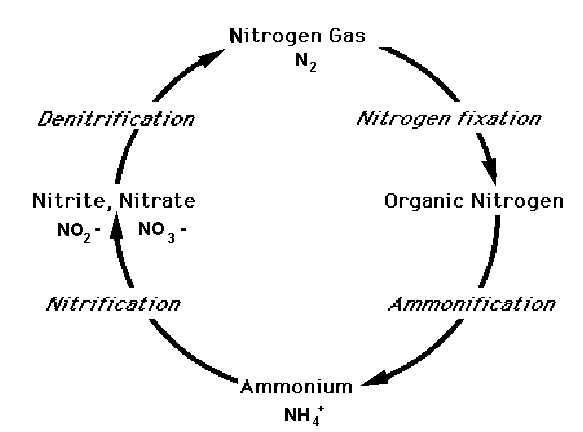
Simplified Nitrogen cycle, italics denote processes and bold the different forms of nitrogen.
PRIMER ON NITROGEN
What is Nitrogen?
Nitrogen is a naturally occurring element that is essential for growth and reproduction in both plants and animals. It is found in amino acids that make up proteins, in nucleic acids, that comprise the hereditary material and life’s blueprint for all cells, and in many other organic and inorganic compounds. In addition, nitrogen comprises about 80% of the Earth's atmosphere.
The Forms of Nitrogen
To appreciate the intricacies of nitrogen loading to coastal waters, some understanding of how nitrogen reacts chemically in the environment may be useful. Nitrogen is an element that can combine with itself or with other elements to make different compounds. For instance nitrogen gas, N2, is a compound made when two nitrogen atoms form a chemical bond. It makes up about 80% of the atmosphere, while oxygen gas, O2, makes up a little less than 20% of the atmosphere. So nitrogen gas is very common and plentiful. However, only a specialized group of bacteria, and industrial fertilizer manufacture, can "fix" this largely inert compound into biologically useful nitrogen compounds. Fertilizer production now exceeds natural nitrogen fixation in making N2 available to the biosphere.
Nitrogen in Living Things
Nitrogen is a component of amino acids and urea. Amino acids are the building blocks of all proteins. Proteins comprise not only structural components such as muscle, tissue and organs, but also enzymes and hormones essential for the functioning of all living things. Urea is a byproduct of protein digestion. We use the term "organic nitrogen" to describe a nitrogen compound that had its origin in living material. The nitrogen in protein and urea is organic nitrogen. Organic nitrogen can enter septic systems as bodily wastes, discarded food material, or as components of cleaning agents.
Ammonification
Many of the transformations of nitrogen are mediated by bacteria that use different forms of nitrogen to fuel some of their metabolic processes. During the processes of decomposition, the nitrogen in proteins is transformed eventually to ammonia, (NH3) or ammonium (NH4+) by certain kinds of bacteria. These processes are called ammonification. Nitrogen leaves the septic tank primarily as ammonium in leachate. Some of the ammonium becomes adsorbed to soil particles and is effectively immobilized from further transport.
Nitrification
Other kinds of bacteria change ammonia to nitrite. And still other kinds of bacteria can change nitrite to nitrate. These processes are called nitrification. Nitrification is an aerobic process. That means nitrification can occur only in the presence of oxygen. The septic tank ammonium that escapes adsorption is subject to nitrification in aerobic leaching field soils.
Denitrification
And yet still other bacterial species can take nitrate and change it back to nitrogen gas through a process called denitrification. Denitrification is an anaerobic process. This means it only takes place when no oxygen or extremely low concentrations of oxygen are available. Denitrification also requires a source of carbon. Some of the nitrate escaping the leaching field soils is denitrified in the unconsolidated soils and groundwater as it flows to the estuary. Determining the amounts of nitrogen lost in this way is an important area of ongoing research.
Simplified Nitrogen Cycle
In summary, nitrogen cycles through the air, water and soils, with many transformations mediated by the actions of specialized bacteria. Some of these transformations require aerobic conditions while others occur only under anaerobic conditions. The best wastewater disposal systems take advantage of the metabolic needs of these bacteria to reduce the amount of nitrogen in the effluent.
