INTRODUCTION TO THE NITROGEN PROBLEM
Today, nearshore waters are among the most fertilized ecosystems on Earth, as a result of the increased delivery of nutrients that have accompanied upland development. Septic systems, fertilizers and atmospheric deposition (acid rain and air pollution) are major contributors of the nutrient,
nitrogen. In many areas nitrogen travels via groundwater to coastal waters where it can dramatically change the functioning of a coastal ecosystem.Generally, nitrogen is in low supply in coastal waters and is therefore considered the nutrient that limits plant growth. This means that given adequate sunlight, the major nutrient that regulates plant growth rates is nitrogen. If the supply of nitrogen increases, some algae (e.g., certain kinds of
phytoplankton and seaweeds, or macroalgae) take up the nitrogen and grow very quickly. Directly or indirectly, their rapid growth affects other organisms in the water."Eutrophication" describes a spectrum of ecological effects caused by an excess of nutrients, that alters the functioning of an aquatic ecosystem. Some rapidly growing algae cloud the water, severely limiting light penetration. Others disproportionately consume
oxygen or decompose in sufficient quantity to deplete the water's oxygen supply, leading to fish kills. These effects degrade the aesthetic qualities of coastal waters that humans treasure, and also threaten recreational uses of waterways and commercial harvest of fish and shellfish.

As human development of the coastal land increases and coastal waters receive even higher inputs of nitrogen, it becomes important to understand the effects of nitrogen loading in coastal waters, and to learn how these can be avoided or mitigated. Widespread concern about the eutrophication of the Earth's coastal waters has led to much research about the sources and effects of nitrogen loading. Communities need this information to make decisions about growth and development.
Nitrogen derives from several sources
Major nitrogen sources in coastal ecosystems include:
atmospheric deposition onto land, freshwaters, the estuary, and onto impervious surfaces such as roads and parking lots; fertilizer use on farms, cranberry bogs, lawns, and golf courses; and wastewater from sewage and on-site septic systems. In many watersheds of southern New England septic systems are the primary contributors to groundwater nitrogen entering estuaries. Tidal exchange can introduce marine nitrogen, but this source is relatively unimportant in embayments receiving high nitrogen loads from the watershed.Some nitrogen is lost on its way to the shoreline
Some nitrogen from atmospheric deposition evaporates and thus returns to the atmosphere. Some nitrogen (mainly ammonium) is adsorbed to soil particles and removed from further transport. Plants take up soil nitrogen (mainly nitrate) and effectively immobilize it. Thus, the
vegetated landscape is important in sequestering nitrogen and slowing its transport to the sea. Some of the nitrogen that enters the soil, subsoils, and groundwater is denitrified and therefore is lost to the system. (Denitrification is a term for a series of chemical reactions carried out by bacteria in which nitrogen in the form of nitrate is converted to inert nitrogen gas.) Any nitrogen that is not lost on its journey through a watershed will be transported eventually to the receiving estuary. The net difference between sources and losses represents the nitrogen loading to the estuary, usually expressed as an amount (e.g., kg) per year.Effects of nitrogen loading can vary among estuaries
Knowing the amount of nitrogen entering an estuary each year is of little use by itself because the amount of nitrogen that is beneficial or harmful to an embayment is site-specific.
Tidal exchange is very important in determining whether nitrogen concentrations can reach excessive levels. Nitrogen entering a well-flushed estuary may quickly disperse to the open sea and therefore contribute little to eutrophication of the estuary. On the other hand, a poorly-flushed embayment may retain its nitrogen load for a long enough time to contribute to eutrophication and disruption of the system. Also, the size of the estuary or embayment will influence the concentration of nitrogen after it enters from the watershed.Nitrogen is rapidly taken up by algae
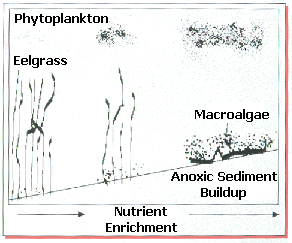
Once in the estuary, nitrogen is rapidly taken up by plant life, especially during warm, sunny days of spring and summer. Algae, both planktonic microscopic forms and seaweeds (
macroalgae), generally respond fastest to the new nitrate seeping into the estuary from the groundwater. In the shallows of the estuary, beds of eelgrass comprise an important fish and shellfish habitat. Eelgrass can take nitrogen from the water through its leaves, but is out-competed by the faster growing algae. Rapidly growing phytoplankton make the water more turbid, decreasing the light necessary for eelgrass growth. Eelgrass becomes increasing starved for light, and more prone to disease and die-off as this stress continues. The grass, which propagates both by runners and seeds, may not be able to replenish itself. The result after several years of diminished growth and lack of replenishment is thinning of eelgrass and its eventual loss from the estuary.How does excessive nitrogen lead to declines of fish and shellfish?
The eelgrass beds support the growth of many juvenile fishes, offering enhanced food and protection. As eelgrass is lost from the estuary, the
fish community suffers dramatic declines in numbers and diversity of fish. As eelgrass is replaced by seaweeds (macroalgae), other indirect effects of excess nitrogen further diminish the ability of the estuary to sustain fish production. In sunlight, the algae produce oxygen during photosynthesis. At night, they use oxygen during respiration. If the weather is cloudy, they cannot produce very much oxygen. During several successive cloudy, hot, windless days in summer, the large numbers of algae can use up all of the dissolved oxygen and cause the suffocation of shellfish and finfish.