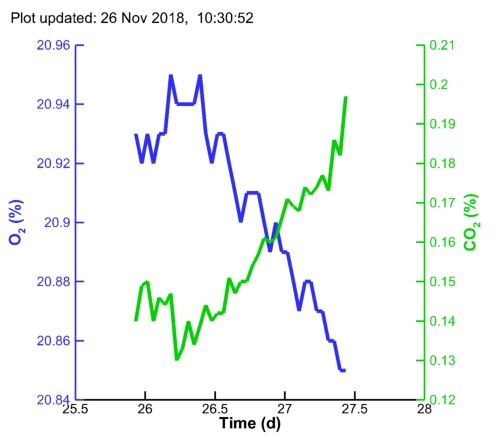
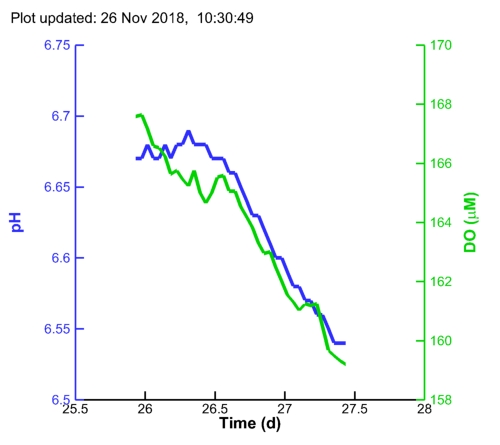
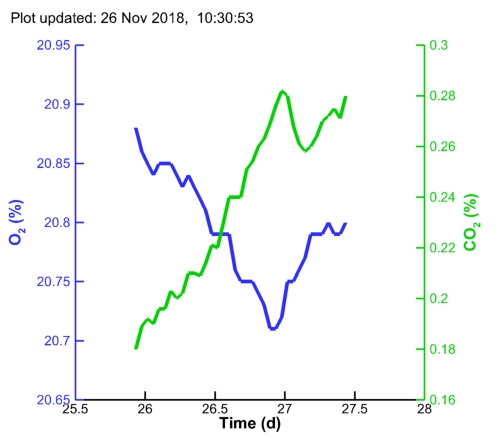
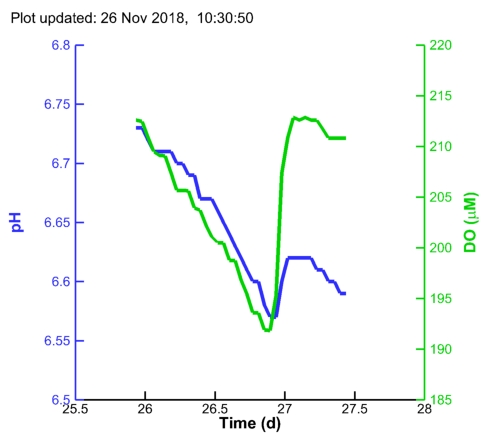
| MC1 pH: |
Trace_2018-10-30_19h52_pH_243632-2353-1178.txt,
Trace_2018-11-12_12h53_pH_243632-2353-1178.txt
|
| MC1 DO: |
Trace_2018-10-30_19h52_DO_242453-02-210052.txt,
Trace_2018-11-12_12h53_DO_242453-02-210052.txt
|
| MC2 pH: |
Trace_2018-10-30_19h53_pH_243632-2353-1176.txt,
Trace_2018-11-12_12h53_pH_243632-2353-1176.txt
|
| MC2 DO: |
Trace_2018-10-30_19h53_DO_242453-02-210053.txt,
Trace_2018-11-12_12h54_DO_242453-02-210053.txt
|