Dissolved Methane Probe Information
Information on the experimental dissolved methane probe construction
and calibration is presented here. Note, as currently configured, this probe as several limiations; namely,
- Oxygen must be present in the liquid for the probe to function. This configuration can not be used to monitor methane
in anaerobic systems (i.e., methanogenic systems). However, there
are solutions to this problem (see F. Yazdian, S. A. Shojaosadati, M.
P. H. M. Nosrati, and K. Malek Khosravi. On-Line Measurement of
Dissolved Methane Concentration During Methane Fermentation in a Loop
Bioreactor. IRANIAN JOURNAL OF CHEMICAL ENGINEERING 28 (4):85-93, 2009).
- While
the sensor has fast response times, gas diffusion across the silicone
membrane causes considerable lag (response time in hours).
The dCH4 probe sensor is a Figaro
TGS2611-C00 metal oxide
semiconductor whose resistence decreases as a function of increasing
methane concentration. By use of a simple voltage divider, methane
concentration can be estimated, as given by this circuit that applies
5V across both the chip heater (RH) and the sensor (RS).
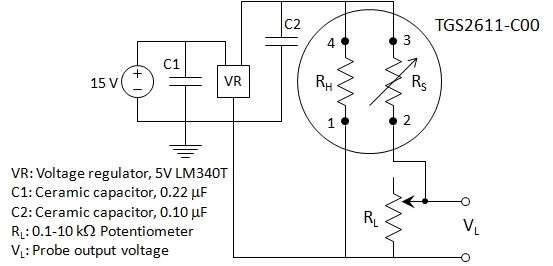
Voltage drop across the load resistor (RL variable resistor adjusted between 5k and 8k ohms) is monitored with a Weeder Technologies WTAIN-M RS232 A2D board. Relationship between voltage, VL, and dissolved methane is obtained via calibration with methane standards (see below).
The
Figaro sensor is packaged into a 1/2" polycarbonate tube that has
Luer-Lok fittings that connect to a 4" piece of silicone tubing.
An o-ring sits in a 1/16" grove cut into the polycarbonate tube,
which holds the senor in place by friction. A GL45 cap with a
modified nylon 1/2" compression union fitting allows the probe to be
appropriately placed in the microcosm. An image of the finished probe
is shown here:
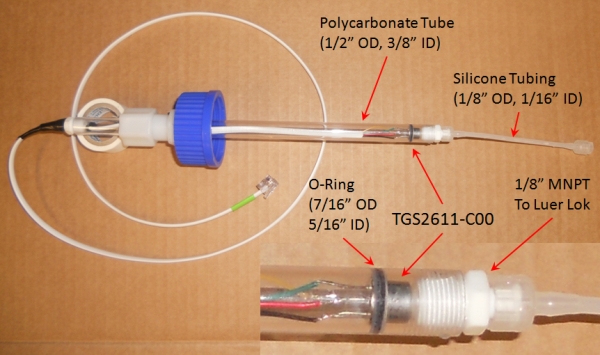
Methane and oxygen diffuse across the silicone tubing and adsorb to the semiconductor in the sensor.
Dissolved methane probes were calibrated by placing probes in micrcosm
reactors filled with DI water and sparged with mixtures of methane and
air. Concentration of methane in gas phase was measured in a manner identical to
that in the main experiment. Because temperature and
transport characteristics strongely influence probe response,
conditions for probe calibration were set as close as possible to those
found in the experimental system.
Probe voltage output as a function of methane gas cocnetration is show
here:
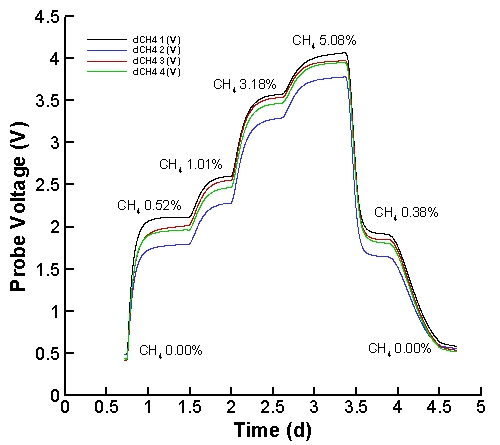
Note, probe response delays are due to transport of methane across
silicone tubing and time required to flush reactor headspace with
desired methane concentraion. All four probes were in the
same reactor, which contained approximately 3 L of DI water and 3 L of
gas
headspace, and gas flow rates varied from 35 to 110 mL min-1.
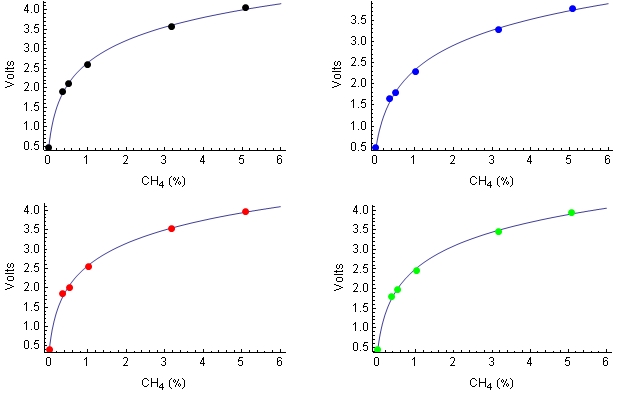
The probe volages were fit to the following logrithmic equation:
v = vM Ln(PCH4
+ kCH4) + v0
where PCH4 is the partial pressure of methane
(%), and the three
parameters were determined by least squares fit to the data presented
above. This
equation fit the data fairly well as shown here:
Recalibration and Tubing Change
After
several days, water accumulates in the silicone tubing, which block
methane transport and causes voltage drops. Larger diameter
silicone tubing is being tested (3/16" OD, 1/8" ID, 2 3/4" long).
Also, resistence of the load resistors (RL) were decreased (to 2 - 3 kohms) and adjusted to that all probes have similar outputs at CH4 concentrations in equilibrium with 1.5% CH4 gas headspace.



